HCOOCH + CH2 + H2O: Understanding the Chemical Reaction and Its Applications
Chemistry is filled with fascinating reactions that combine various compounds to form new substances. One such reaction involves the combination of HCOOCH + CH2 + H2O. Each of these compounds plays a unique role in organic and inorganic chemistry. In this article, we will explore the HCOOCH + CH2 + H2O reaction in depth—examining its structure, chemical properties, possible reaction mechanisms, industrial applications, and significance in research and environmental chemistry.
1. Introduction to HCOOCH + CH2 + H2O
The combination HCOOCH + CH2 + H2O may appear simple, but it represents an interesting intersection of formate esters, methylene groups, and water molecules. These substances are fundamental in organic synthesis and biochemical processes.
Let’s briefly break down what each chemical component represents:
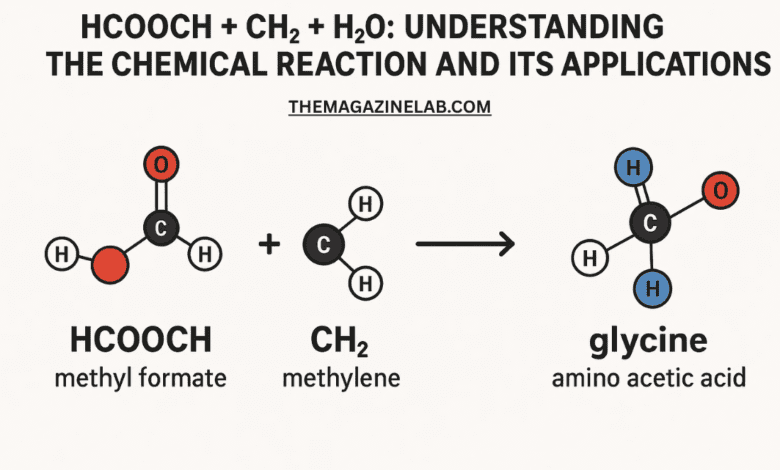
- HCOOCH — A formate ester, also known as methyl formate (HCOOCH3) when written completely, is an organic compound commonly used as an intermediate in chemical synthesis.
- CH2 — Represents a methylene group, a reactive species in many organic reactions such as polymerization and addition reactions.
- H2O — Water, the universal solvent, plays a critical role in hydrolysis, hydration, and various chemical equilibria.
When these three compounds are combined under suitable conditions, they can form new products depending on the type of reaction involved—commonly hydrolysis, condensation, or addition reactions.
2. Chemical Nature of HCOOCH (Methyl Formate)
The compound HCOOCH (better represented as HCOOCH3) is the methyl ester of formic acid. It is a colorless, flammable liquid with a pleasant, ether-like odor.
Key Properties:
- Molecular Formula: C2H4O2
- Molar Mass: 60.05 g/mol
- Boiling Point: 32°C
- Density: 0.97 g/cm³
Methyl formate is widely used as an intermediate in the production of formamide, dimethylformamide (DMF), and other industrial solvents. It can undergo hydrolysis in the presence of water (H2O) to yield methanol (CH3OH) and formic acid (HCOOH), a reaction that demonstrates its chemical reactivity.
3. Understanding the Role of CH2 in HCOOCH + CH2 + H2O
The CH2 group, also known as methylene, is one of the most versatile intermediates in organic chemistry. It can appear as a neutral molecule, a carbene (CH2:), or as a part of larger organic structures.
When combined with compounds like HCOOCH and H2O, the CH2 group can facilitate carbon–carbon bond formation, substitution, or addition reactions. In catalytic or polymerization reactions, CH2 can help build longer carbon chains or modify existing organic structures.
In the context of HCOOCH + CH2 + H2O, CH2 can participate in:
- Hydration reactions, leading to the formation of alcohols.
- Addition reactions, resulting in new organic intermediates.
- Reformation reactions, where water may influence the oxidation state of carbon atoms.
4. The Function of H2O in HCOOCH + CH2 + H2O
Water (H2O) is not just a solvent but an active participant in many chemical processes. In reactions involving esters like HCOOCH, water commonly acts as a reactant in hydrolysis reactions.
Hydrolysis of HCOOCH:
When HCOOCH3 (methyl formate) reacts with water, it forms:HCOOCH3+H2O→HCOOH+CH3OHHCOOCH_3 + H_2O → HCOOH + CH_3OHHCOOCH3+H2O→HCOOH+CH3OH
This reaction breaks the ester bond, yielding formic acid and methanol. The presence of CH2 in such a system could promote additional side reactions, depending on whether the methylene group is stabilized or free as a carbene.
5. Reaction Pathways Involving HCOOCH + CH2 + H2O
The HCOOCH + CH2 + H2O system can lead to several reaction pathways, depending on the reaction conditions such as temperature, pH, and catalysts. Let’s consider a few possibilities:
a. Hydrolysis Pathway
If the reaction occurs in an aqueous environment:HCOOCH3+H2O→HCOOH+CH3OHHCOOCH_3 + H_2O → HCOOH + CH_3OHHCOOCH3+H2O→HCOOH+CH3OH
Here, CH2 may not directly react but can influence the overall mechanism by stabilizing intermediates or acting as a bridge in more complex systems.
b. Addition Reaction Pathway
When CH2 acts as a reactive carbene:HCOOCH3+CH2→CH3CH2OCHOHCOOCH_3 + CH_2 → CH_3CH_2OCHOHCOOCH3+CH2→CH3CH2OCHO
This could potentially yield a substituted ether or an alcohol derivative upon hydration.
c. Condensation Pathway
Under certain catalytic conditions, CH2 and HCOOCH can undergo condensation, possibly leading to formaldehyde derivatives or larger esters. The introduction of H2O in such systems usually leads to hydrolysis or partial oxidation.
6. Industrial and Practical Applications of HCOOCH + CH2 + H2O
The combination of formate esters (HCOOCH), methylene species (CH2), and water is of great importance in industrial and laboratory chemistry. Here are some practical applications and uses:
a. Production of Solvents
Hydrolysis of methyl formate produces methanol, an essential solvent and fuel additive. The resulting formic acid is used in tanning, cleaning, and as a preservative.
b. Intermediate in Chemical Synthesis
CH2 can help extend carbon chains in organic synthesis, making the HCOOCH + CH2 + H2O system valuable for producing aldehydes, alcohols, and esters.
c. Fuel and Energy Research
Methyl formate is being investigated as a potential biofuel component due to its clean combustion and renewable synthesis routes. Reactions involving CH2 and water can be part of reformative processes that release hydrogen or methanol.
d. Environmental Chemistry
Formic acid, one of the products of the hydrolysis reaction, is biodegradable and environmentally friendly, making the HCOOCH + CH2 + H2O system useful in green chemistry.
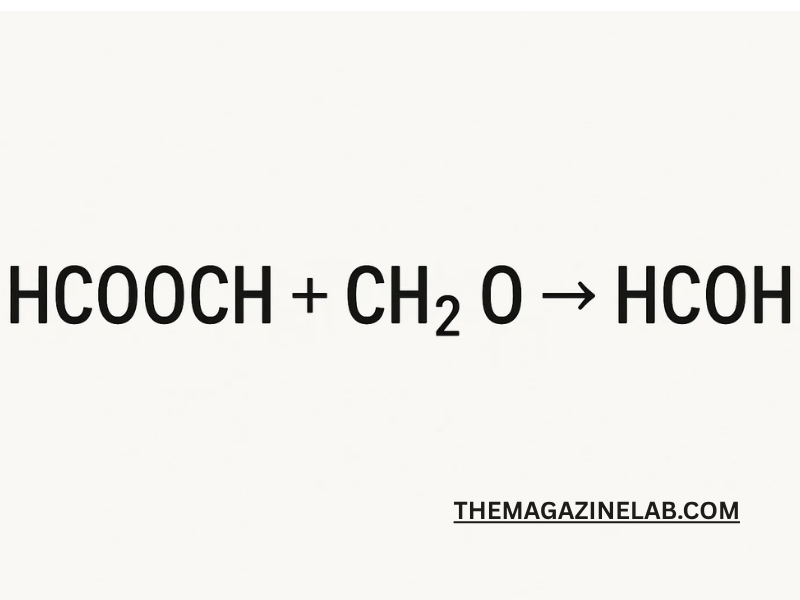
7. Theoretical Importance of HCOOCH + CH2 + H2O in Organic Mechanisms
The reaction between HCOOCH, CH2, and H2O is also of theoretical interest in studying reaction mechanisms.
- Carbene Insertion: CH2 can act as a reactive carbene inserting itself into C–H or C–O bonds of HCOOCH.
- Hydrolysis Kinetics: Understanding how water interacts with esters like HCOOCH helps chemists design more efficient catalysts for esterification or hydrolysis.
- Energy Pathways: Computational chemists use reactions like these to model energy barriers and transition states, improving predictions for catalytic performance.
8. Safety and Handling of HCOOCH, CH2, and H2O
While H2O is harmless, HCOOCH (methyl formate) and CH2 (methylene species) require careful handling.
Safety Notes:
- HCOOCH is highly flammable and can form explosive mixtures with air.
- CH2 is a transient and reactive species that must be generated in controlled environments.
- Reactions should be performed under proper ventilation and temperature control to prevent decomposition or unwanted by-products.
9. Laboratory Demonstration of HCOOCH + CH2 + H2O Reaction
In an academic setting, chemists might demonstrate this reaction to illustrate hydrolysis or ester cleavage mechanisms.
Example Procedure:
- Mix methyl formate (HCOOCH3) with distilled water (H2O).
- Add a few drops of acid or base catalyst (e.g., HCl or NaOH).
- Observe the production of formic acid and methanol.
If a CH2 source is introduced, such as diazomethane (CH2N2), new compounds like extended esters or ethers may form depending on the reaction medium.
10. Environmental Impact and Sustainability
Reactions involving HCOOCH + CH2 + H2O can contribute to sustainable chemistry by:
- Generating biodegradable products like formic acid.
- Supporting methanol-based energy cycles.
- Offering low-emission alternatives in industrial synthesis.
Methyl formate, for instance, is being studied as a replacement for ozone-depleting solvents, while methanol serves as a clean-burning fuel.
Conclusion
The HCOOCH + CH2 + H2O reaction exemplifies the intricate interplay between organic compounds, reactive intermediates, and water. Through hydrolysis, addition, and condensation pathways, this system can yield important products like formic acid, methanol, and extended carbon-chain compounds.
From industrial solvents to renewable fuels, and from theoretical chemistry to sustainable practices, understanding the reaction dynamics of HCOOCH + CH2 + H2O provides valuable insights into the chemical processes shaping modern science and industry.
In short, this reaction is not merely an academic curiosity—it’s a cornerstone example of how simple molecules can interact to produce compounds vital for life, energy, and technology.